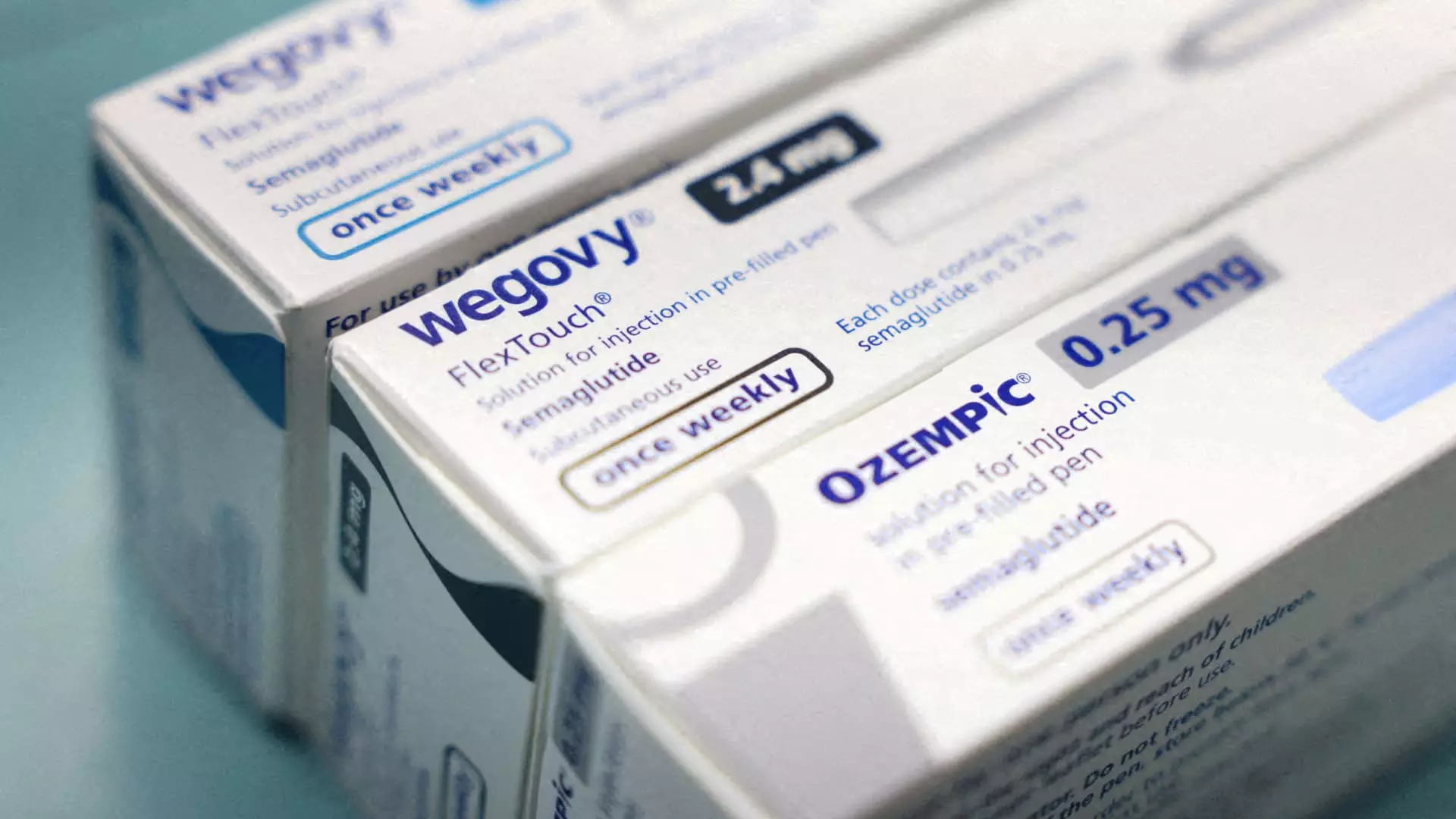
In a significant development for the pharmaceutical industry, the U.S. Food and Drug Administration (FDA) has announced that the prolonged shortage of Novo Nordisk’s highly sought-after weight loss and diabetes treatment medications, Wegovy and Ozempic, has officially come to an end. This resolution arrives after a critical two-year period marked by heightened demand and limited availability of these injections, which contain the active ingredient semaglutide. The implications of this announcement extend far beyond just the reinvigoration of pharmaceutical supplies; they reverberate throughout healthcare practices, patient welfare, and the financial landscapes of competing companies.
Understanding the Shortage and Its Impacts
Since their introduction, semaglutide-based medications have gained significant attention, primarily due to their effectiveness in promoting weight loss and managing diabetes. The soaring popularity was met with production challenges, plunging the market into a severe shortage that began in 2022. With patients desperately seeking access to these treatments, many turned to compounding pharmacies, which filled the gap by providing unregulated versions of Wegovy and Ozempic. The reliance on these compounded medications illuminated a critical vulnerability in the healthcare supply chain, ultimately risking patient safety for the sake of accessible treatment options.
The FDA’s declaration that the supply chain for FDA-approved semaglutide is now stable addresses the immediate market failures but raises new concerns. Compounding pharmacies, which had been operating in a gray legal area, will be forced to cease the production of these unregulated alternatives. This transition from unapproved compounded drugs to a regulated market could result in short-term accessibility issues as pharmacies adjust to the new supply dynamics.
Novo Nordisk’s stock witnessed a notable 5% surge following the FDA’s announcement, a clear indication of investor confidence returning to the company. Conversely, Hims & Hers, a telehealth provider that relied on offering compounded versions of these treatments, faced a staggering drop in shares of over 25%. This contrasting market reaction highlights the broader implications of the FDA’s resolution on competition within the healthcare sector. With a competitive horizon that analysts predict could yield a market value exceeding $150 billion by 2030, the stakes have never been higher, as both Novo Nordisk and Eli Lilly vie for dominance.
Eli Lilly, offering the alternative tirzepatide through its products Zepbound and Mounjaro, is also impacted by the resolution of semaglutide shortages. Just months prior, the FDA had declared tirzepatide shortages resolved, and now Novo Nordisk appears positioned to directly compete in an increasingly lucrative weight-loss drug market. The ongoing competition between these two pharmaceutical giants is not only vital for their corporate growth but also essential for patients seeking effective treatments.
Novo Nordisk’s executive vice president has articulated the importance of this FDA resolution not just in terms of market mechanics but also in terms of patient health. The lack of access to FDA-approved medications can lead patients toward dubious alternatives that could pose serious health risks. As faces of integrity in the healthcare industry, pharmaceutical companies must commit to delivering reliable and safe treatment options that patients can trust.
However, the challenge lies ahead: while Ozempic is often covered by health insurance plans, options like Wegovy are not universally included, particularly under Medicare. This financial barrier can dissuade patients from pursuing effective weight loss treatments, thus complicating the broader narrative about accessibility in healthcare. Ensuring that effective medications are both available and affordable will be pivotal moving forward.
The FDA’s recent announcement signifies a definitive turning point not only for Novo Nordisk but also for the entire landscape of weight loss and diabetes treatment. While the resolution of semaglutide shortages marks a newfound stability in supply chains, it also creates overarching questions regarding accessibility, competition, and patient health moving into the future. As the pharmaceutical sector navigates this new terrain, the emphasis must remain firmly on patient welfare, ensuring that effective treatments become accessible and safe for those in need.